Enhanced Normal Modes Analysis with Bio3D
Lars Skjaerven, Xin-Qiu Yao, Guido Scarabelli & Barry J. Grant
Bio3D_nma.RmdBackground
Bio3D1 is an R package that provides interactive tools for structural bioinformatics . The primary focus of Bio3D is the analysis of biomolecular structure, sequence and simulation data (Grant et al. 2006).
Normal mode analysis (NMA) is one of the major simulation techniques used to probe large-scale motions in biomolecules. Typical application is for the prediction of functional motions in proteins. Version 2.0 of the Bio3D package now includes extensive NMA facilities (Skjaerven et al. 2015). These include a unique collection of multiple elastic network model (ENM) force-fields (see Example 1 below), automated ensemble analysis methods (Example 2), variance weighted NMA (Example 3), and NMA with user-defined force fields (Example 4). Here we demonstrate the use of these new features with working code that comprise complete executable examples2.
Requirements
Detailed instructions for obtaining and installing the Bio3D package on various platforms can be found in the Installing Bio3D Vignette available both on-line and from within the Bio3D package. In addition to Bio3D the MUSCLE multiple sequence alignment program (available from the muscle home page) must be installed on your system and in the search path for executables. Please see the installation vignette for further details.
Example 1: Basic Normal Mode Analysis
Example 1A: Normal mode calculation
Normal mode analysis (NMA) of a single protein structure can be carried out by providing a PDB object to the function nma(). In the code below we first load the Bio3D package and then download an example structure of hen egg white lysozyme (PDB id 1hel) with the function read.pdb(). Finally the function nma() is used perform the normal mode calculation:
## Note: Accessing on-line PDB filemodes <- nma(pdb)
## Building Hessian... Done in 0.015 seconds.
## Diagonalizing Hessian... Done in 0.076 seconds.A short summary of the returned nma object contained within the new variable modes can be obtained by simply calling the function print():
print(modes)
##
## Call:
## nma.pdb(pdb = pdb)
##
## Class:
## VibrationalModes (nma)
##
## Number of modes:
## 387 (6 trivial)
##
## Frequencies:
## Mode 7: 0.018
## Mode 8: 0.019
## Mode 9: 0.024
## Mode 10: 0.025
## Mode 11: 0.028
## Mode 12: 0.029
##
## + attr: modes, frequencies, force.constants, fluctuations,
## U, L, xyz, mass, temp, triv.modes, natoms, callThis reveals the function call resulting in the nma object along with the total number of stored normal modes. For PDB id 1hel there are 129 amino acid residues, and thus \(387\) modes (\(3*129=387\)) in this object. The first six modes are so-called trivial modes with zero frequency and correspond to rigid-body rotation and translation. The frequency of the next six lowest-frequency modes is also printed.
Note that the returned nma object consists of a number of attributes listed on the +attr: line. These attributes contain the detailed results of the calculation and their complete description can be found on the nma() functions help page accessible with the command: help(nma). To get a quick overview of the results one can simply call the plot() function on the returned nma object. This will produce a summary plot of (1) the eigenvalues, (2) the mode frequencies, and (3) the atomic fluctuations (See Figure 1).
plot(modes, sse=pdb)
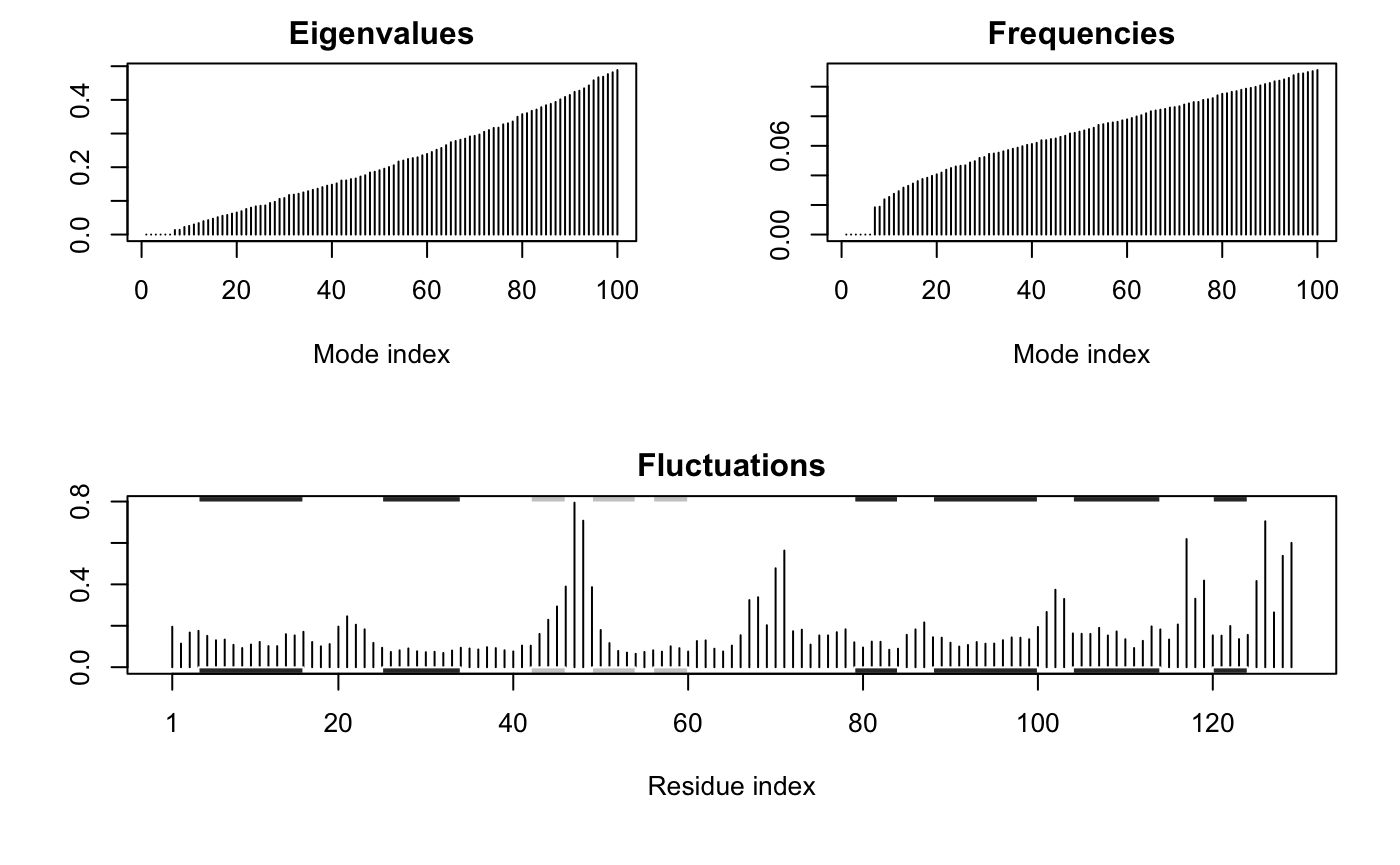
Summary plot of NMA results for hen egg white lysozyme (PDB id 1hel). The optional sse=pdb argument provided to plot.nma() results in a secondary structure schematic being added to the top and bottom margins of the fluctuation plot (helices black and strands gray). Note the larger fluctuations predicted for loop regions.
Example 1B: Specifying a force field
The main Bio3D normal mode analysis function, nma(), requires a set of coordinates, as obtained from the read.pdb() function, and the specification of a force field describing the interactions between constituent atoms. By default the calpha force field originally developed by Konrad Hinsen is utilized (Hinsen et al. 2000). This employs a spring force constant differentiating between nearest-neighbor pairs along the backbone and all other pairs. The force constant function was parameterized by fitting to a local minimum of a crambin model using the AMBER94 force field. However, a number of additional force fields are also available, as well as functionality for providing customized force constant functions. Full details of available force fields can be obtained with the command help(load.enmff). With the code below we briefly demonstrate their usage along with a simple comparison of the modes obtained from two of the most commonly used force fields:
help(load.enmff)
# Calculate modes with various force fields modes.a <- nma(pdb, ff="calpha") modes.b <- nma(pdb, ff="anm") modes.c <- nma(pdb, ff="pfanm") modes.d <- nma(pdb, ff="reach") modes.e <- nma(pdb, ff="sdenm") # Root mean square inner product (RMSIP) r <- rmsip(modes.a, modes.b)
plot(r, xlab="ANM", ylab="C-alpha FF")
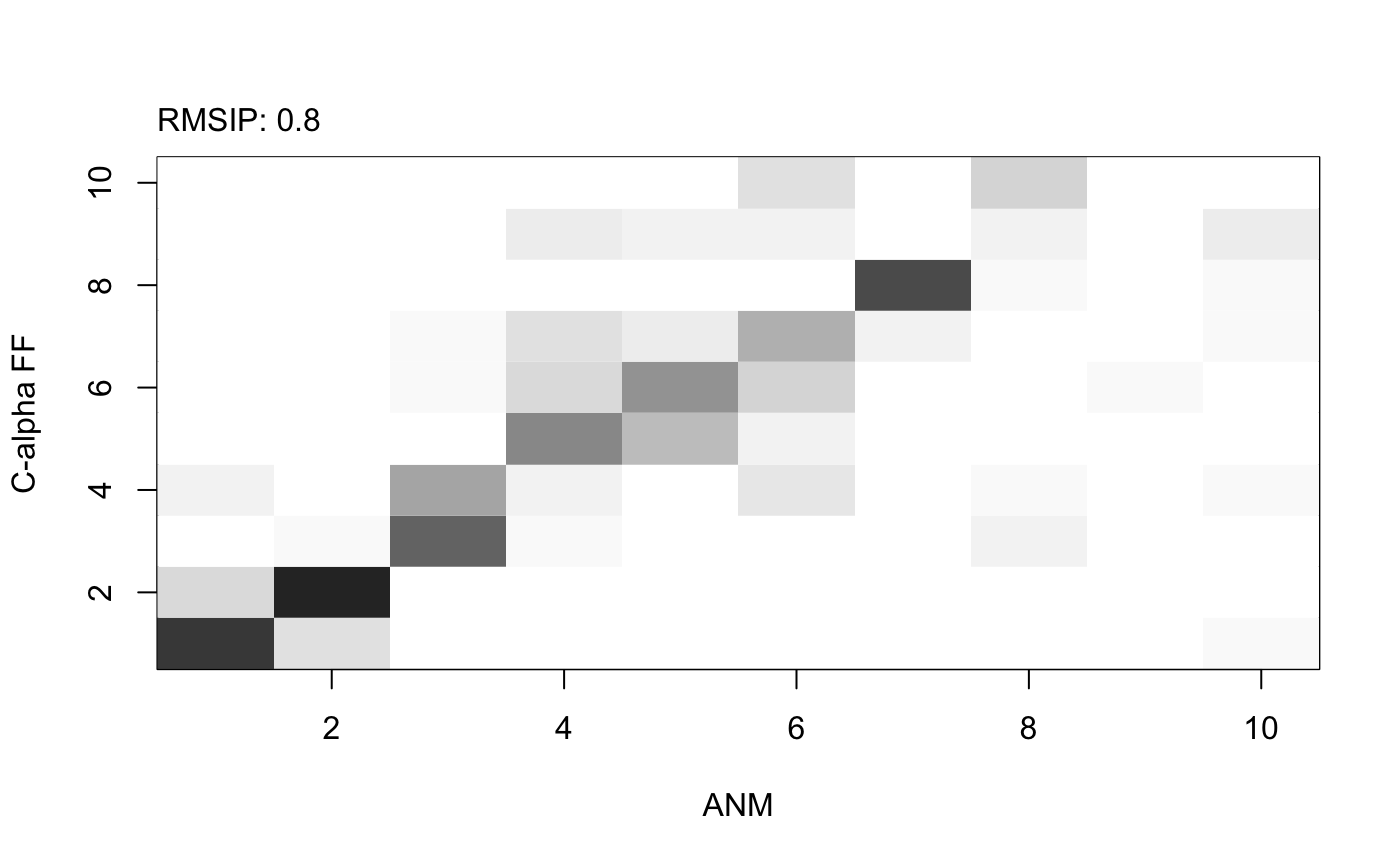
Analysis of mode similarity between modes obtained from the ANM and calpha force fields by calculating mode overlap and root mean square inner product (RMSIP) with function rmsip(). An RMSIP value of 1 depicts identical directionality of the two mode subspaces.
Example 1C: Normal mode analysis of the GroEL subunit
Bio3D includes a number of functions for analyzing and visualizing the normal modes. In the example below we illustrate this functionality on the GroEL subunit. GroEL is a multimeric protein consisting of 14 identical subunits organized in three distinct domains inter-connected by two hinge regions facilitating large conformational changes.
We will investigate the normal modes through (1) mode visualization to illustrate the nature of the motions; (2) cross-correlation analysis to determine correlated regions; (3) deformation analysis to measure the local flexibility of the structure; (4) overlap analysis to determine which modes contribute to a given conformational change; and (5) domain analysis to identify regions of the protein moving as rigid parts.
Calculate the normal modes
In the code below we download a structure of GroEL (PDB-id 1sx4) and use atom.select() to select one of the 14 subunits prior to the call to nma():
# Download PDB, calcualte normal modes of the open subunit pdb.full <- read.pdb("1sx4") pdb.open <- trim.pdb(pdb.full, atom.select(pdb.full, chain="A")) modes <- nma(pdb.open)
Mode visualization
With Bio3D you can visualize the normal modes either by generating a trajectory file which can be loaded into a molecular viewer program (e.g. VMD or PyMOL), or through a vector field representation in PyMOL. Both functions, mktrj.nma() and pymol.modes(), takes an nma object as input in addition to the mode index specifying which mode to visualize:
# Make a PDB trajectory mktrj(modes, mode=7) # Vector field representation (see Figure 3.) pymol(modes, mode=7)

Visualization of the first non-trivial mode of the GroEL subunit. Visualization is provided through a trajectory file (left), or vector field representation (right).
Cross-correlation analysis
Function dccm.nma() calculates the cross-correlation matrix of the nma object. Function plot.dccm() will draw a correlation map, and 3D visualization of correlations is provided through function pymol.dccm():
# Calculate the cross-correlation matrix cm <- dccm(modes)
# Plot a correlation map with plot.dccm(cm) plot(cm, sse=pdb.open, contour=F, col.regions=bwr.colors(20), at=seq(-1,1,0.1) )
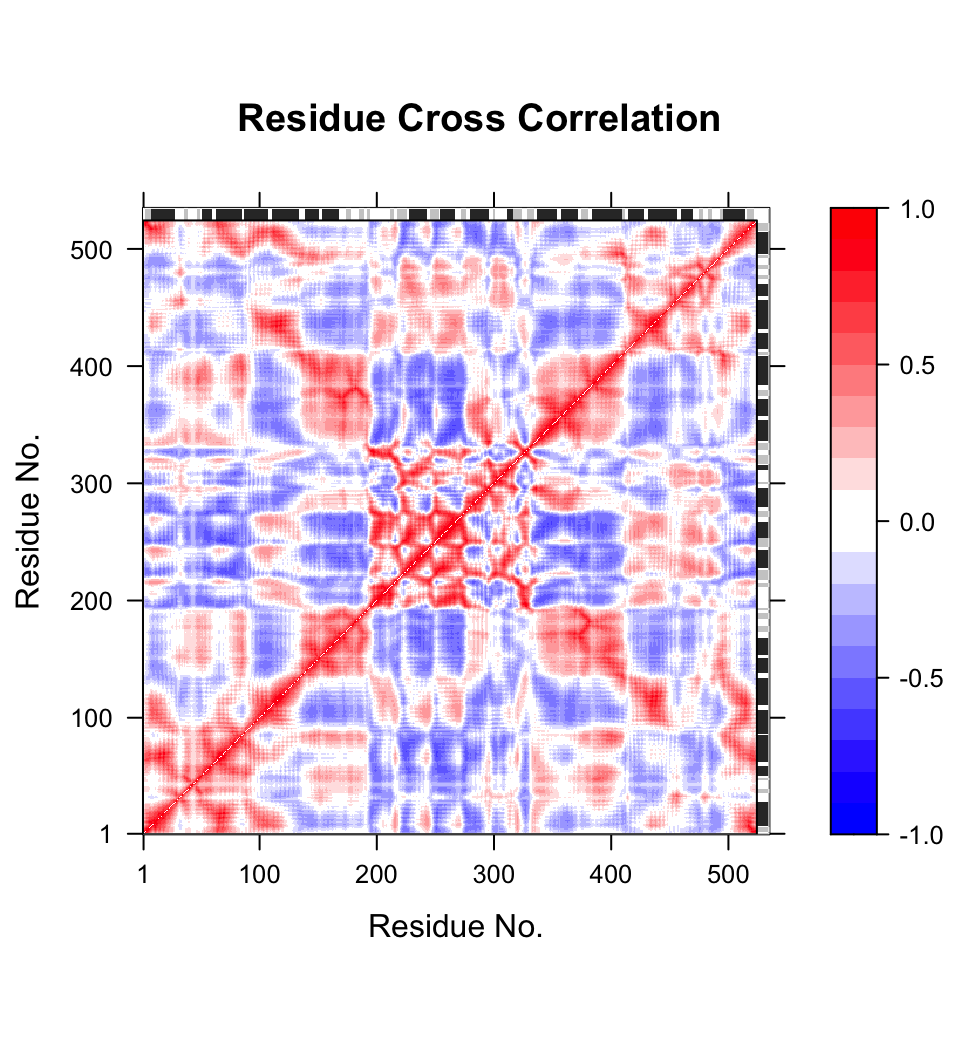
Correlation map revealing correlated and anti-correlated regions in the protein structure.
# View the correlations in the structure (see Figure 5.) pymol(cm, pdb.open, type="launch")
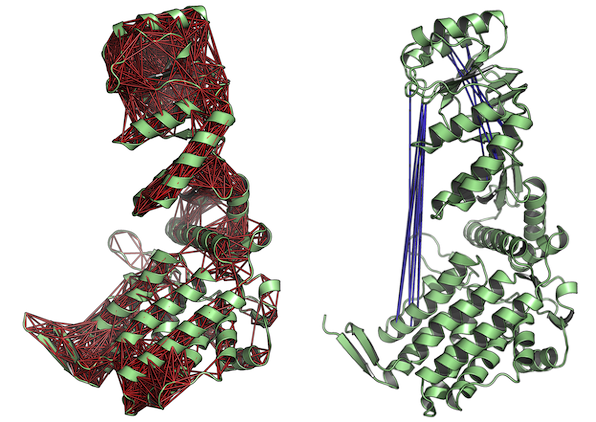
Correlated (left) and anti-correlated (right) residues depicted with red and blue lines, respectively. The figures demonstrate the output of function pymol.dccm().
Fluctuation and Deformation analysis
Deformation analysis provides a measure for the amount of local flexibility in the protein structure - i.e. atomic motion relative to neighboring atoms. It differs from fluctuations (e.g. RMSF values) which provide amplitudes of the absolute atomic motion. Below we calculate deformation energies (with deformation.nma()) and atomic fluctuations (with fluct.nma()) of the first three modes and visualize the results in PyMOL:
# Deformation energies defe <- deformation.nma(modes) defsums <- rowSums(defe$ei[,1:3]) # Fluctuations flucts <- fluct.nma(modes, mode.inds=seq(7,9))
# Write to PDB files (see Figure 6.) write.pdb(pdb=NULL, xyz=modes$xyz, file="R-defor.pdb", b=defsums) write.pdb(pdb=NULL, xyz=modes$xyz, file="R-fluct.pdb", b=flucts)
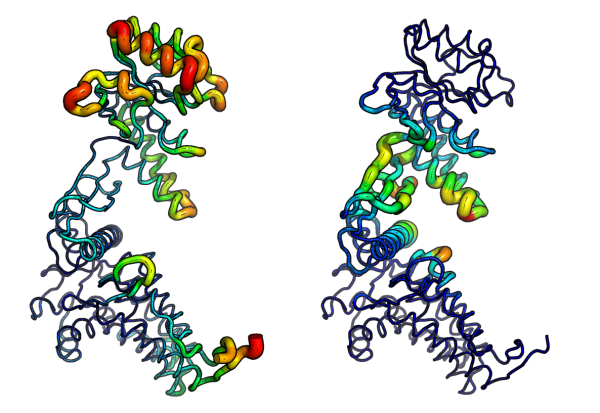
Atomic fluctuations (left) and deformation energies (right) visualized in PyMOL.
Overlap analysis
Finally, we illustrate overlap analysis to compare a conformational difference vector with the normal modes to identify which modes contribute to a given conformational change (i.e. the difference between the open and closed state of the GroEL subunit).
# Closed state of the subunit pdb.closed <- trim.pdb(pdb.full, atom.select(pdb.full, chain="H")) # Align closed and open PDBs aln <- struct.aln(pdb.open, pdb.closed, max.cycles=0) pdb.closed$xyz <- aln$xyz # Caclulate a difference vector xyz <- rbind(pdb.open$xyz[aln$a.inds$xyz], pdb.closed$xyz[aln$a.inds$xyz]) diff <- difference.vector(xyz) # Calculate overlap oa <- overlap(modes, diff)
plot(oa$overlap, type='h', xlab="Mode index", ylab="Squared overlap", ylim=c(0,1)) points(oa$overlap, col=1) lines(oa$overlap.cum, type='b', col=2, cex=0.5) text(c(1,5)+.5, oa$overlap[c(1,5)], c("Mode 1", "Mode 5"), adj=0)
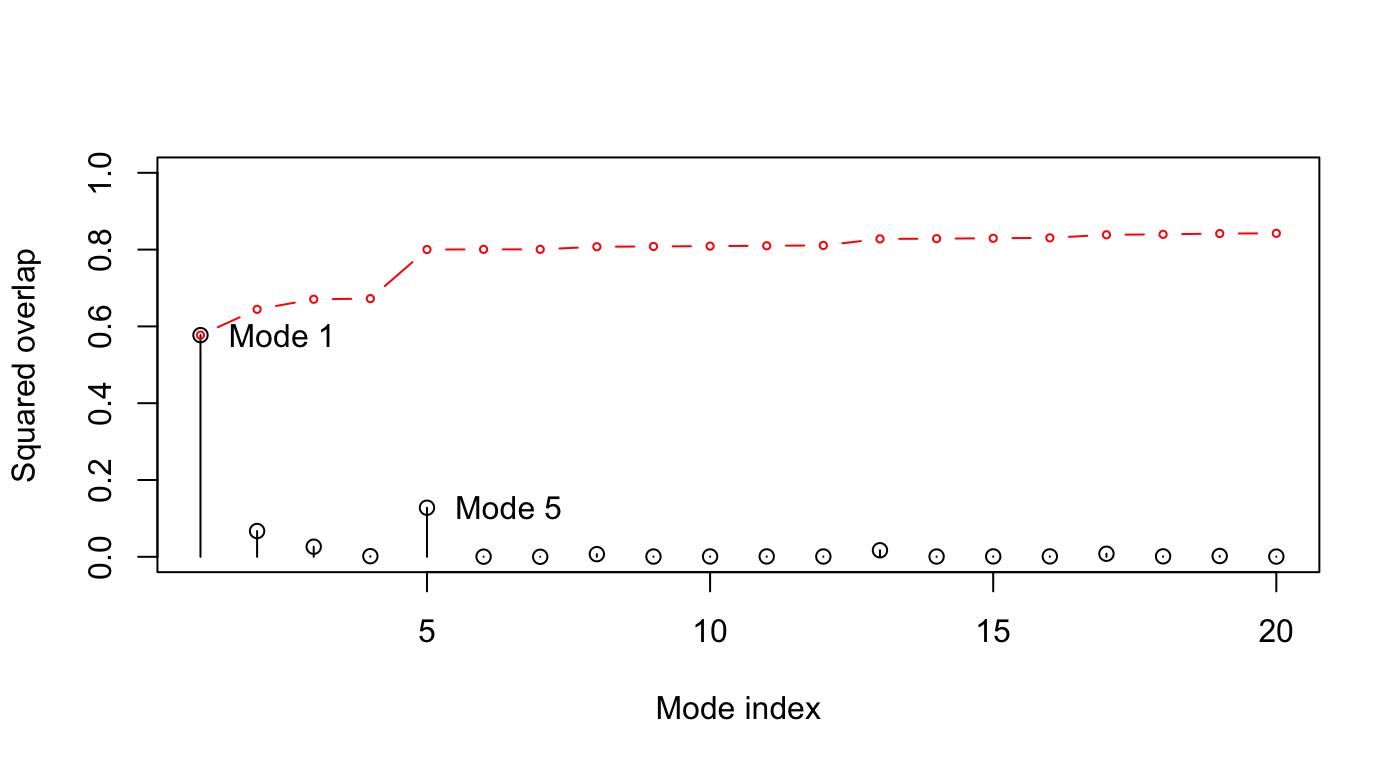
Overlap analysis between the modes of the open subunit and the conformational difference vector between the closed-open state.
Domain analysis with GeoStaS
Identification of regions in the protein that move as rigid bodies is facilitated with the implementation of the GeoStaS algorithm (Romanowska, Nowinski, and Trylska 2012). Below we demonstrate the use of function geostas() on an nma object, and an ensemble of X-ray structures. See help(geostas) for more details and further examples.
GeoStaS with NMA: Starting from the calculated normal modes, geostas generates a conformational ensemble by interpolating along the eigenvectors of the first 5 normal modes of the GroEL subunit. This ensemble is then used to search for rigid domains:
# Run geostas to find domains gs <- geostas(modes, k=4)
# Write NMA trajectory with domain assignment mktrj(modes, mode=7, chain=gs$grps)
GeoStaS with X-ray structure ensemble: Alternatively the same analysis can be performed on an ensemble of X-ray structures obtained from the PDB:
# Define the ensemble PDB-ids ids <- c("1sx4_[A,B,H,I]", "1xck_[A-B]", "1sx3_[A-B]", "4ab3_[A-B]") # Download and split PDBs by chain ID raw.files <- get.pdb(ids, "groel_pdbs", gzip=TRUE) files <- pdbsplit(raw.files, ids, path = "groel_pdbs") # Align and superimpose coordinates pdbs <- pdbaln(files, fit=TRUE) # Run geostast to find domains gs <- geostas(pdbs, k=4)
# Plot a atomic movement similarity matrix plot(gs, margin.segments=gs$grps, contour=FALSE)
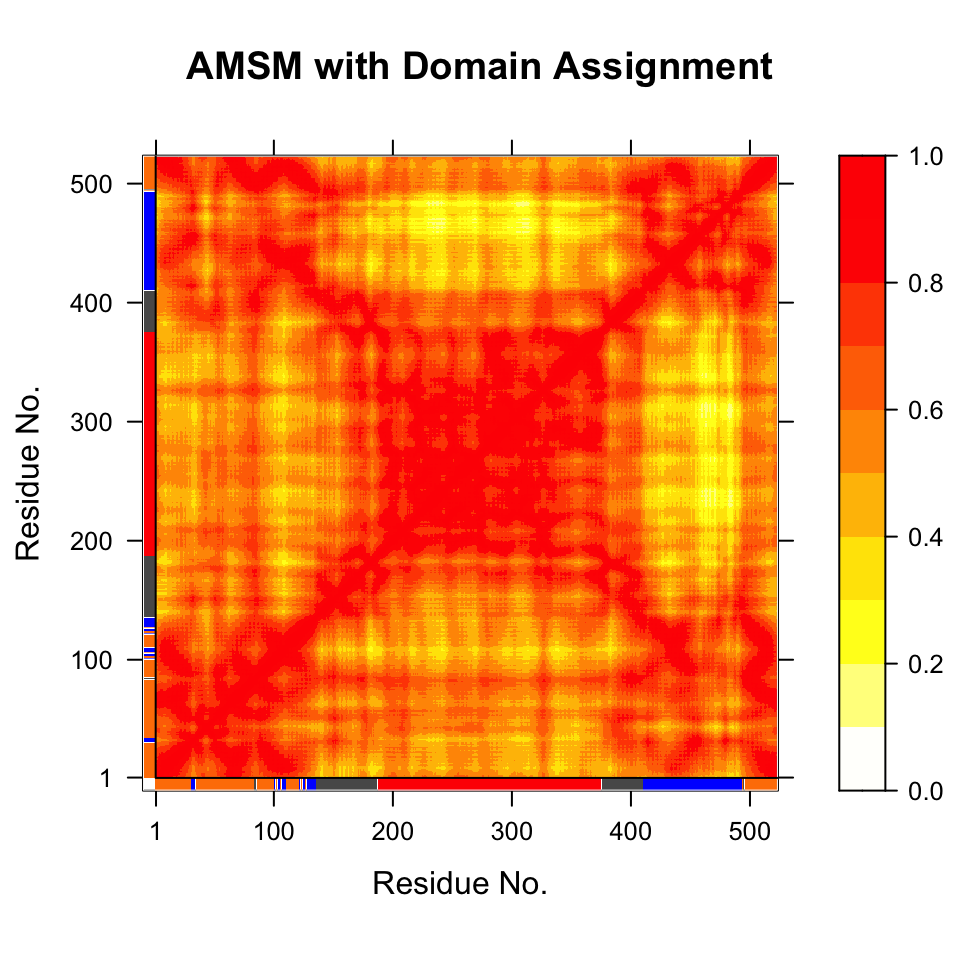
Atomic movement similarity matrix with domain annotation.
# Principal component analysis gaps.pos <- gap.inspect(pdbs$xyz) xyz <- fit.xyz(pdbs$xyz[1, gaps.pos$f.inds], pdbs$xyz[, gaps.pos$f.inds], fixed.inds=gs$fit.inds, mobile.inds=gs$fit.inds) pc.xray <- pca.xyz(xyz)
# Visualize PCs with colored domains (chain ID) mktrj(pc.xray, pc=1, chain=gs$grps)
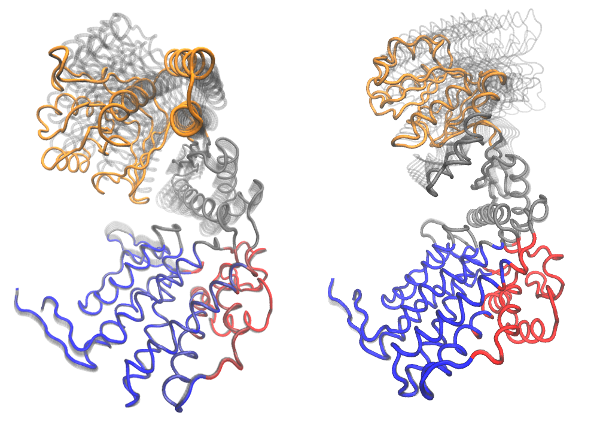
Visualization of domain assignment obtained from function geostas() using (left) an ensemble of X-ray structures and (right) NMA.
Example 2: All-atom normal mode analysis (ENM)
The coarse-grained NMA approach used above omits potentially important inter-atomic interactions in the normal modes calculations. To provide a more detailed model Bio3D now offers an ENM using all atoms. We have recently shown that this all-atom approach provides normal mode vectors with an improved agreement to normal modes calculated with the full empiric force field (i.e. the Amber99SB force field) (Yao, Skjaerven, and Grant 2016). The followning examples demonstrates some of the capabilities with all-atom ENM in Bio3D.
Example 2A: Dihydrofolate reductase
In the example below we load a structure of E.coli Dihydrofolate reductase (DHFR) and call function aanma() on the pdb object to build the all-atom elastic network and calculate the normal modes. In this particular case we use argument outmodes="calpha" to specify the diagonalization of the effective Hessian for the calpha atoms only (Hinsen et al. 2000). In this way we can obtain normal mode vectors only for the calpha atoms which facilitates e.g. comparison with coarse grained NMA.
# read e.coli DHFR pdb <- read.pdb("1rg7")
## Note: Accessing on-line PDB file# keep only protein + methotrexate pdb <- trim(pdb, "notwater") # calculate all-atom NMA with ENM, output calpha only m.aa <- aanma(pdb, outmodes="calpha")
## Building Hessian... Done in 1.141 seconds.
## Extracting effective Hessian.. Done in 23.753 seconds.
## Diagonalizing Hessian... Done in 0.141 seconds.# write summary m.aa
##
## Call:
## aanma.pdb(pdb = pdb, outmodes = "calpha")
##
## Class:
## VibrationalModes (nma)
##
## Number of modes:
## 477 (6 trivial)
##
## Frequencies:
## Mode 7: 0.02
## Mode 8: 0.022
## Mode 9: 0.023
## Mode 10: 0.026
## Mode 11: 0.027
## Mode 12: 0.03
##
## + attr: modes, frequencies, force.constants, fluctuations,
## U, L, xyz, mass, temp, triv.modes, natoms, callWe can compare the directionality of mode vectors with results obtained from coarse grained NMA using function rmsip():
# compare with c-alpha modes m.ca <- nma(pdb)
## Building Hessian... Done in 0.016 seconds.
## Diagonalizing Hessian... Done in 0.139 seconds.rmsip(m.aa, m.ca)
## $overlap
## b1 b2 b3 b4 b5 b6 b7 b8 b9 b10
## a1 0.773 0.002 0.062 0.001 0.070 0.001 0.021 0.003 0.001 0.003
## a2 0.040 0.662 0.001 0.008 0.108 0.011 0.000 0.005 0.004 0.017
## a3 0.041 0.061 0.117 0.407 0.194 0.002 0.000 0.002 0.002 0.040
## a4 0.055 0.092 0.103 0.253 0.148 0.133 0.041 0.019 0.013 0.037
## a5 0.011 0.012 0.402 0.017 0.115 0.147 0.008 0.061 0.047 0.004
## a6 0.001 0.005 0.039 0.022 0.140 0.092 0.187 0.081 0.001 0.088
## a7 0.000 0.000 0.080 0.003 0.024 0.029 0.049 0.045 0.197 0.011
## a8 0.001 0.006 0.002 0.052 0.029 0.293 0.001 0.081 0.082 0.079
## a9 0.003 0.049 0.022 0.027 0.017 0.002 0.011 0.002 0.018 0.062
## a10 0.031 0.007 0.000 0.004 0.001 0.030 0.074 0.002 0.116 0.035
##
## $rmsip
## [1] 0.8128787
##
## attr(,"class")
## [1] "rmsip"Supplying argument outmodes="noh" will result in that the entire all-atom (heavy atoms) Hessian matrix is diagonalized. This will output a modes object containing all 3840 mode vectors facilitating e.g. trajetory output of all-atom NMA:
# all-atom NMA, but output all atoms m.noh <- aanma(pdb, outmodes="noh")
## Building Hessian... Done in 1.226 seconds.
## Diagonalizing Hessian... Done in 71.076 seconds.# output trajectory of first modes mktrj(m.noh, mode = 7, pdb=pdb)

Visualization of the first non-trivial mode of DHFR using an all-atom elastic network model for the normal mode calculation (function aanma()). Ligand MTX is shown in sapce filling representation to illustrate it’s present in the model and the normal modes calculation.
Example 2B: Reducing the computational load
Although all-atom ENM provides normal modes with better agreement to Amber modes it is also a far more computational expensive approach (compare 20.7 and 0.2 seconds for the example above). To speed up the calculation of all-atom ENM Bio3D provides functionality for the rotation-translation block based approximation (Durand, Trinquier, and Sanejouand 1994) (Tama et al. 2000). In this method, each residue is assumed to be a rigid body (or block) that has only rotational and translational degrees of freedom. Intra-residue deformation is thus ignored. This approach significantly speeds up the calculation of the normal modes while the results are largely identical:
# use rotation and translation of blocks m.rtb <- aanma(pdb, rtb=TRUE)
## Building Hessian... Done in 1.132 seconds.
## Extracting effective Hessian with RTB.. Done in 3.682 seconds.
## Diagonalizing Hessian with RTB... Done in 0.15 seconds.rmsip(m.aa, m.rtb)
## $overlap
## b1 b2 b3 b4 b5 b6 b7 b8 b9 b10
## a1 1 0.000 0.000 0.000 0 0 0 0.000 0.000 0.000
## a2 0 0.999 0.000 0.000 0 0 0 0.000 0.000 0.000
## a3 0 0.000 0.999 0.000 0 0 0 0.000 0.000 0.000
## a4 0 0.000 0.000 0.999 0 0 0 0.000 0.000 0.000
## a5 0 0.000 0.000 0.000 1 0 0 0.000 0.000 0.000
## a6 0 0.000 0.000 0.000 0 1 0 0.000 0.000 0.000
## a7 0 0.000 0.000 0.000 0 0 1 0.000 0.000 0.000
## a8 0 0.000 0.000 0.000 0 0 0 0.999 0.000 0.000
## a9 0 0.000 0.000 0.000 0 0 0 0.000 0.999 0.000
## a10 0 0.000 0.000 0.000 0 0 0 0.000 0.000 0.992
##
## $rmsip
## [1] 0.9995298
##
## attr(,"class")
## [1] "rmsip"Bio3D also provides a reduced atom model in which only a selection of all heavy atoms is used to build the ENM. More specifically, three to five atoms per residue constitute the model. Here the N, CA, C atoms represent the protein backbone, and zero (for Gly), one (for Lys, Ser, Cys, Pro, Ala, Met, Phe, and Tyr) or two (for the remaining amino acids) selected side chain atoms represent the side chain (selected based on side chain size and the distance to CA). This reduced-atom ENM has significantly improved computational efficiency and similar prediction accuracy with respect to the all-atom ENM.
# use reduced-atom ENM m.red <- aanma(pdb, reduced=TRUE)
## Building Hessian... Done in 0.362 seconds.
## Extracting effective Hessian.. Done in 3.867 seconds.
## Diagonalizing Hessian... Done in 0.141 seconds.rmsip(m.aa, m.red)
## $overlap
## b1 b2 b3 b4 b5 b6 b7 b8 b9 b10
## a1 0.878 0.063 0.017 0.003 0.001 0.000 0.000 0.009 0.012 0.001
## a2 0.078 0.594 0.280 0.006 0.001 0.004 0.000 0.001 0.006 0.004
## a3 0.002 0.306 0.649 0.004 0.001 0.004 0.001 0.004 0.002 0.004
## a4 0.006 0.000 0.006 0.930 0.000 0.005 0.004 0.004 0.005 0.000
## a5 0.002 0.001 0.001 0.001 0.900 0.002 0.062 0.002 0.001 0.001
## a6 0.000 0.008 0.001 0.005 0.013 0.853 0.070 0.001 0.000 0.001
## a7 0.000 0.001 0.001 0.000 0.052 0.089 0.786 0.000 0.012 0.006
## a8 0.003 0.001 0.000 0.000 0.000 0.000 0.006 0.631 0.003 0.233
## a9 0.001 0.003 0.021 0.003 0.001 0.002 0.010 0.034 0.715 0.108
## a10 0.009 0.001 0.000 0.000 0.000 0.005 0.000 0.188 0.029 0.191
##
## $rmsip
## [1] 0.9468653
##
## attr(,"class")
## [1] "rmsip"The two approximations can also be combined to further decrease the computational expense:
# use both 4-bead and RTB approach m.rr <- aanma(pdb, reduced=TRUE, rtb=TRUE)
## Building Hessian... Done in 0.284 seconds.
## Extracting effective Hessian with RTB.. Done in 2.421 seconds.
## Diagonalizing Hessian with RTB... Done in 0.149 seconds.Example 3: Ensemble normal mode analysis
The analysis of multiple protein structures (e.g. a protein family) can be accomplished with the nma.pdbs() function.3 This will take aligned input structures, as generated by the pdbaln() function for example, and perform NMA on each structure collecting the results in manner that facilitates the interpretation of similarity and dissimilarity trends in the structure set. Here we will analyze a collection of Dihydrofolate reductase (DHFR) structures with low sequence identity (Example 3A) and large set of closely related transducin heterotrimeric G protein family members (Example 3B).
Example 3A: Dihydrofolate reductase
In the following code we collect 9 bacterial DHFR structures of 4 different species from the protein databank (using get.pdb()) with sequence identity down to 27% (see the call to function seqidentity() below), and align these with pdbaln():
# Select bacterial DHFR PDB IDs ids <- c("1rx2_A", "1rx4_A", "1rg7_A", "3jw3_A", "3sai_A", "1df7_A", "4kne_A", "3fyv_X", "3sgy_B") # Download and split by chain ID raw.files <- get.pdb(ids, path="raw_pdbs") files <- pdbsplit( raw.files, ids ) # Alignment of structures pdbs <- pdbaln(files)
# Sequence identity summary( c(seqidentity(pdbs)) )
## Min. 1st Qu. Median Mean 3rd Qu. Max.
## 0.2680 0.3460 0.3960 0.5265 0.9940 1.0000The pdbs object now contains aligned C-alpha atom data, including Cartesian coordinates, residue numbers, residue types, and B-factors. The sequence alignment is also stored by default to the FASTA format file ‘aln.fa’ (to view this you can use an alignment viewer such as SEAVIEW, see Requirements section above). Function nma.pdbs() will calculate the normal modes of each protein structures stored in the pdbs object. The normal modes are calculated on the full structures as provided by object pdbs. With the default argument rm.gaps=TRUE unaligned atoms are omitted from output in accordance with common practice (Fuglebakk, Echave, and Reuter 2012).
# NMA on all structures modes <- nma(pdbs)
The modes object of class enma contains aligned normal mode data including fluctuations, RMSIP data, and aligned eigenvectors. A short summary of the modes object can be obtain by calling the function print(), and the aligned fluctuations can be plotted with function plot():
print(modes)
##
## Call:
## nma.pdbs(pdbs = pdbs)
##
## Class:
## enma
##
## Number of structures:
## 9
##
## Attributes stored:
## - Root mean square inner product (RMSIP)
## - Aligned atomic fluctuations
## - Aligned eigenvectors (gaps removed)
## - Dimensions of x$U.subspace: 456x450x9
##
## Coordinates were aligned prior to NMA calculations
##
## + attr: fluctuations, rmsip, U.subspace, L, full.nma, xyz,
## call## Extracting SSE from pdbs$sse attributelegend("topleft", col=unique(col), lty=1, legend=c("E.Coli", "B.Anthracis", "M.Tubercolosis", "S.Aureus"))
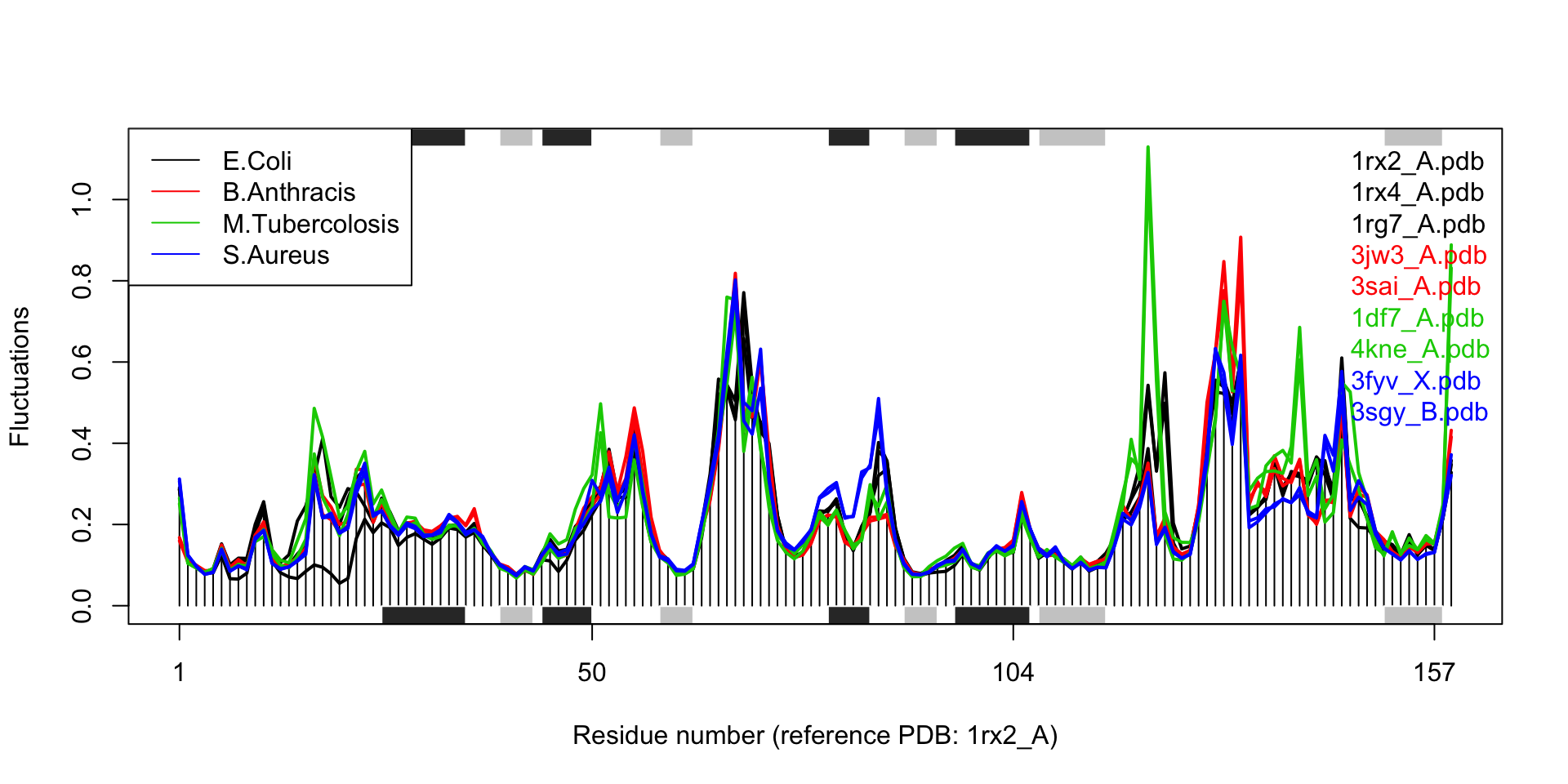
Results of ensemble NMA on four distinct bacterial species of the DHFR enzyme.
# Alternatively, one can use 'rm.gaps=FALSE' to keep the gap containing columns modes <- nma.pdbs(pdbs, rm.gaps=FALSE)
Cross-correlation analysis can be easily performed and the results contrasted for each member of the input ensemble. Below we calculate and plot the correlation matrices for each structure and then output correlations present only in all input structures.
# Calculate correlation matrices for each structure cij <- dccm(modes)
# Determine correlations present only in all 9 input structures cij.all <- filter.dccm(cij$all.dccm, cutoff.sims=9, cutoff.cij = 0) plot.dccm(cij.all, main="Consensus Residue Cross Correlation")
Example 3B: Transducin
In this section we will demonstrate the use of nma.pdbs() on the example transducin family data that ships with the Bio3D package. This can be loaded with the command data(transducin) and contains an object pdbs consisting of aligned C-alpha coordinates for 53 transducin structures from the PDB as well their annotation (in the object annotation) as obtained from the pdb.annotate() function. Note that this data can be generated from scratch by following the Comparative Structure Analysis with Bio3D Vignette available both on-line and from within the Bio3D package.
# Load data data(transducin) pdbs <- transducin$pdbs annotation <- transducin$annotation # Find gap positions gaps.res <- gap.inspect(pdbs$ali) gaps.pos <- gap.inspect(pdbs$xyz)
# Calculate normal modes of the 53 structures modes <- nma.pdbs(pdbs, ncore=4)
# Make fluctuation plot plot(modes, col=annotation[, "color"], pdbs=pdbs)
## Extracting SSE from pdbs$sse attribute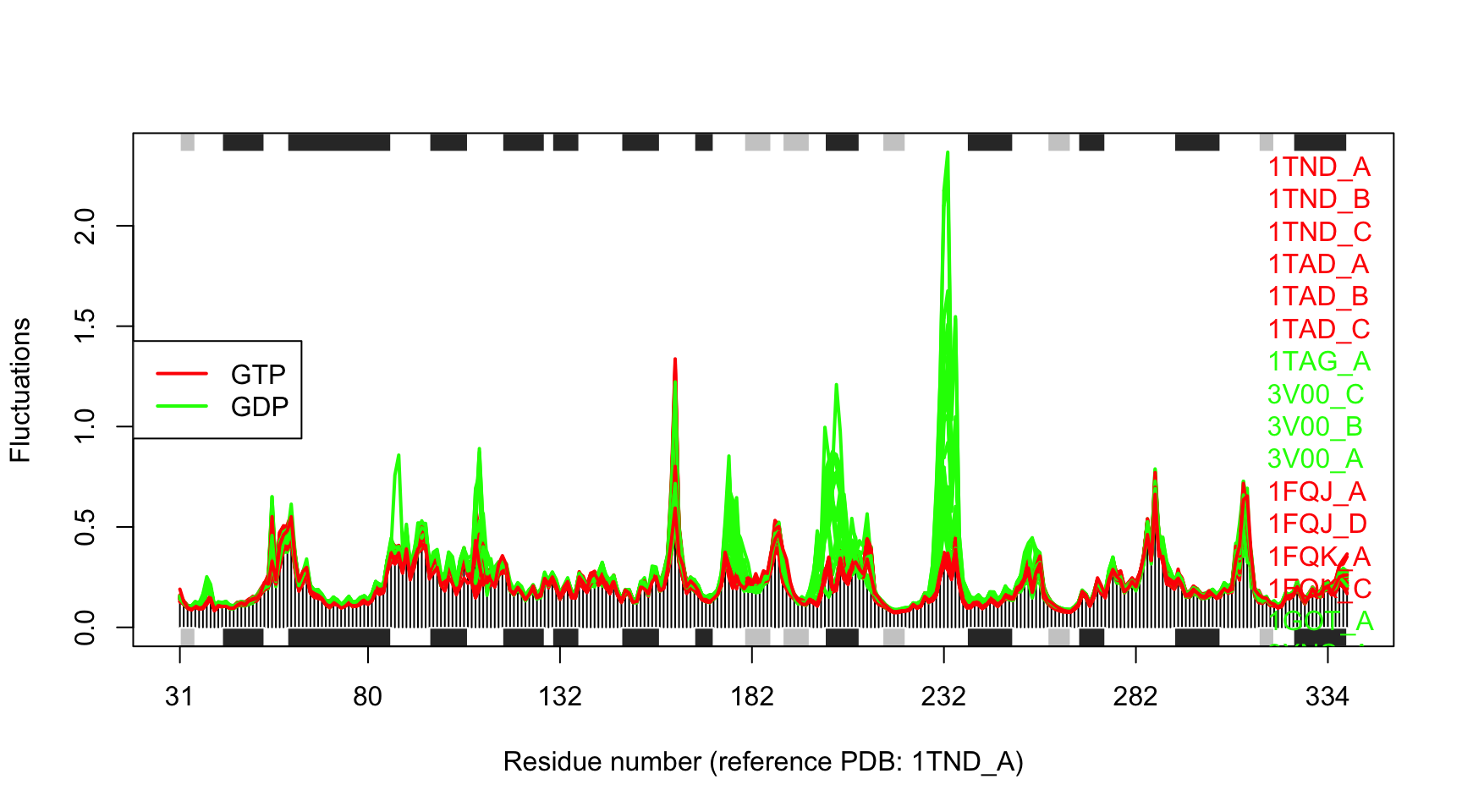
Structural dynamics of transducin. The calculation is based on NMA of 53 structures: 28 GTP-bound (red), and 25 GDP-bound (green).
The similarity of structural dynamics is calculated by RMSIP based on the 10 lowest frequency normal modes. The rmsip values are pre-calculated in the modes object and can be accessed through the attribute modes$rmsip. As a comparison, we also calculate the root mean square deviation (RMSD) of all pair-wise structures:
# Plot a heat map with clustering dendogram ids <- substr(basename(pdbs$id), 1, 6) heatmap((1-modes$rmsip), labRow=annotation[, "state"], labCol=ids, symm=TRUE)
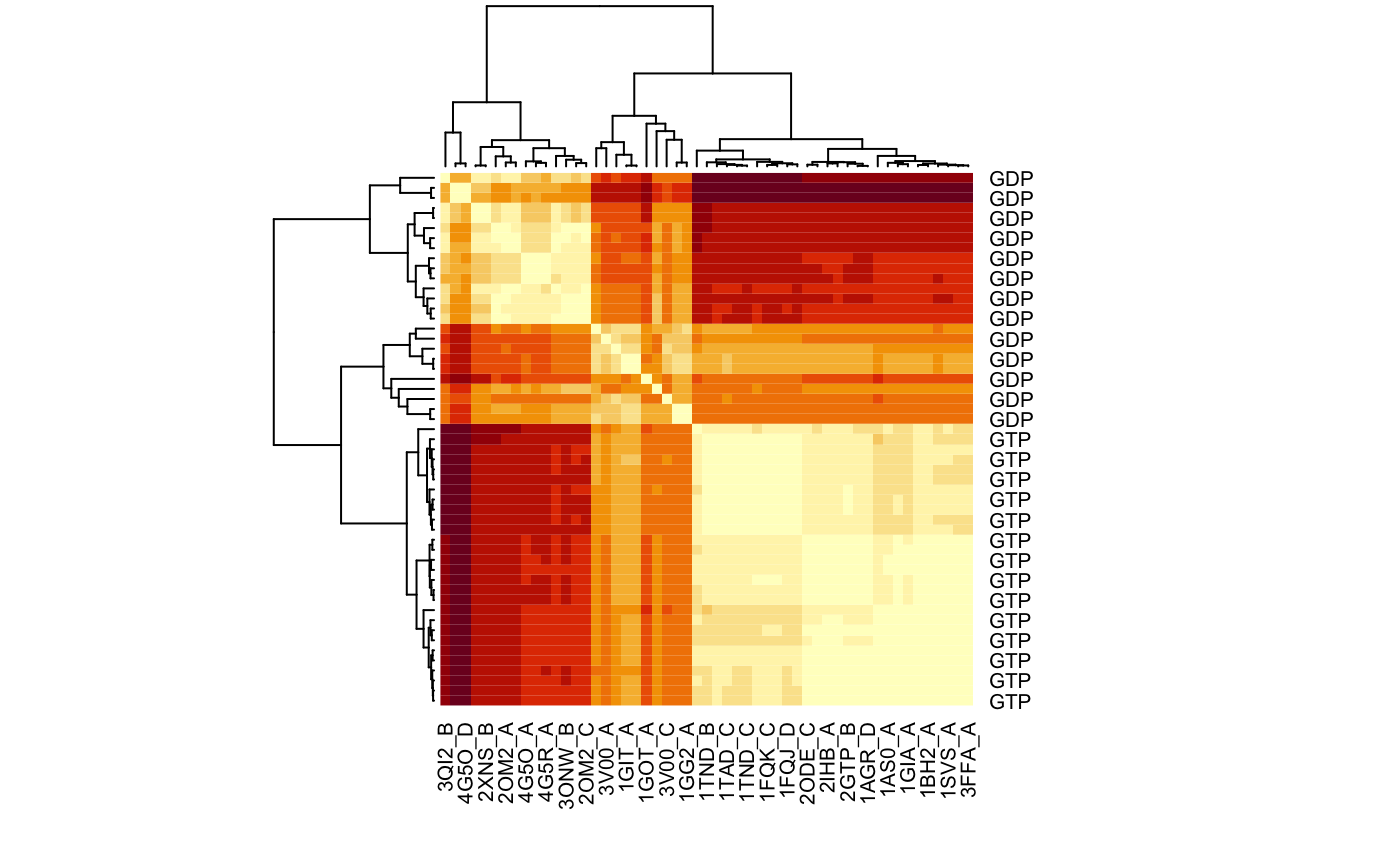
RMSIP matrix of the transducin family.
# Calculate pair-wise RMSD values rmsd.map <- rmsd(pdbs$xyz, a.inds=gaps.pos$f.inds, fit=TRUE) heatmap(rmsd.map, labRow=annotation[, "state"], labCol=ids, symm=TRUE)

RMSD matrix of the transducin family.
Example 3C: All-atom Ensemble NMA
We can also apply all-atom ENMs to the ensemble NMA approach using function aanma.pdbs(). Note the need for function read.all() to obtain an all-atom version of the pdbs object.
# Select bacterial DHFR PDB IDs ids <- c("1rx2_A", "1df7_A", "4kne_A", "3sgy_B") # Download and split by chain ID raw.files <- get.pdb(ids, path="raw_pdbs") files <- pdbsplit( raw.files, ids ) # Read and align structures aln <- pdbaln(files) pdbs <- read.all(aln)
# calculate modes modes <- aanma(pdbs, rtb=TRUE)
## Fitting pdb structuresdone
##
## Details of Scheduled Calculation:
## ... 4 input structures
## ... storing 450 eigenvectors for each structure
## ... dimension of x$U.subspace: ( 456x450x4 )
## ... coordinate superposition prior to NM calculation
## ... aligned eigenvectors (gap containing positions removed)
## ... rotation-translation block (RTB) approximation will be applied
## ... estimated memory usage of final 'eNMA' object: 6.3 Mb# Make fluctuation plot plot(modes, pdbs=pdbs)
## Extracting SSE from pdbs$sse attribute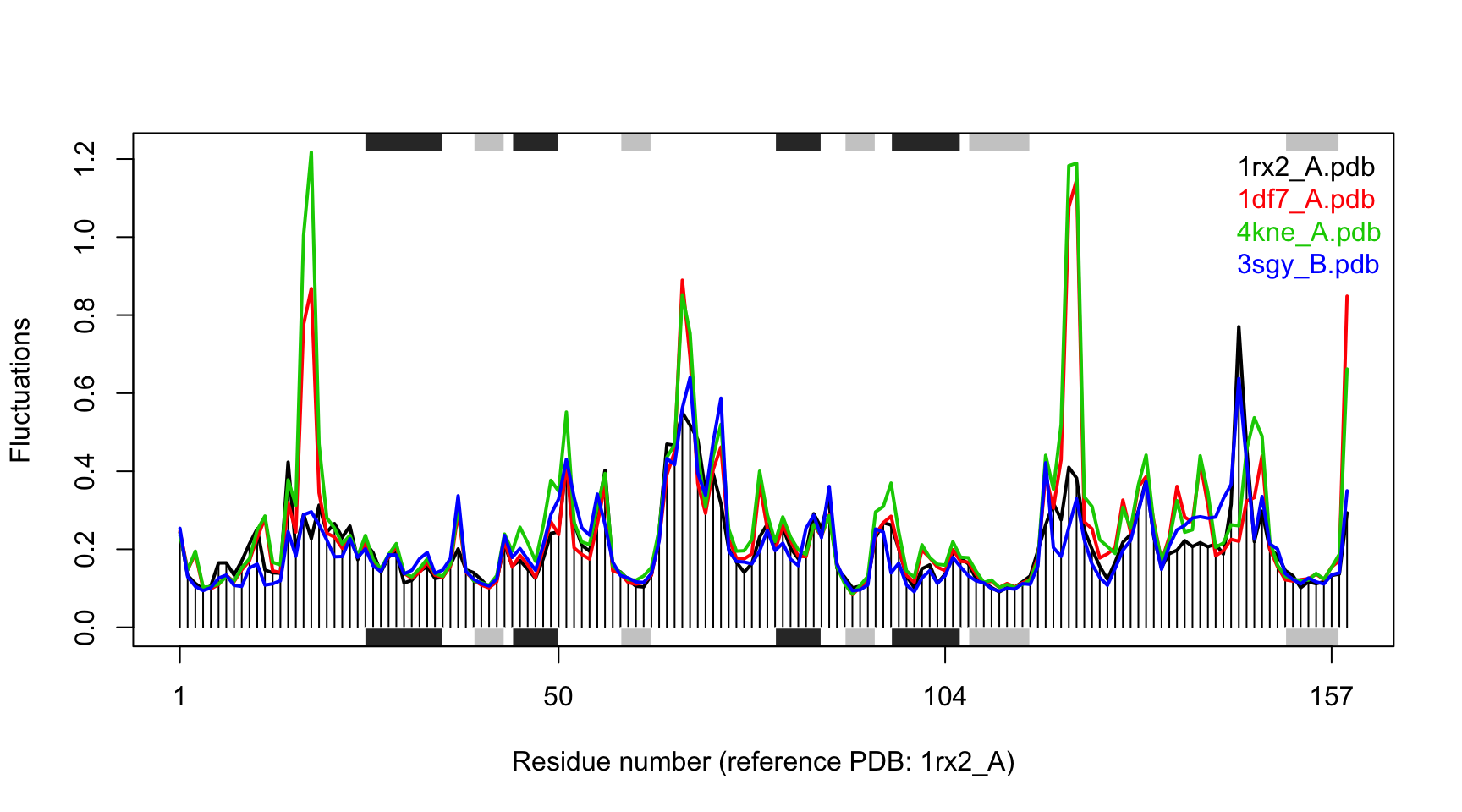
Fluctuation plot of 4 E.coli DHFR structures calculated using all-atom ENM.
Example 4: Variance weighted normal mode analysis
In this example we illustrate an approach of weighting the pair force constants based on the variance of the inter atomic distances obtained from an ensemble of structures (e.g. available X-ray structures). The motivation for such variance-weighting is to reduce the well known dependence of the force constants on the one structure upon which they are derived (Tama and Sanejouand 2001).
Example 4A: GroEL
We first calculate the normal modes of both the closed and open state of the GroEL subunit, and we illustrate the difference in the agreement towards the observed conformational changes (characterized by X-ray and EM studies). We will then use an ensemble of X-ray/EM structures as weights to the pair-force constants.
# Define the ensemble PDB-ids ids <- c("1sx4_[A,B,H,I]", "1xck_[A-B]", "1sx3_[A-B]", "4ab3_[A-B]") # Download and split PDBs by chain ID raw.files <- get.pdb(ids, "groel_pdbs", gzip=TRUE) files <- pdbsplit(raw.files, ids, path = "groel_pdbs") # Align and superimpose coordinates pdbs <- pdbaln(files, fit=TRUE)
Calculate normal modes
Next we will calculate the normal modes of the open and closed conformational state. They are stored at indices 1 and 5, respectively, in our pdbs object. Use the pdbs2pdb() to fetch the pdb objects which is needed for the input to nma().
# Inspect gaps gaps.res <- gap.inspect(pdbs$ali) gaps.pos <- gap.inspect(pdbs$xyz) # Access PDB objects pdb.list <- pdbs2pdb(pdbs, inds=c(1,5,9), rm.gaps=TRUE)
Note that we are here using the argument rm.gaps=TRUE to omit residues in gap containing columns of the alignment. Consequently, the resulting three pdb objects we obtain will have the same lengths (523 residues), which is convenient for subsequent analysis.
Overlap analysis
Use overlap analysis to determine the agreement between the normal mode vectors and the conformational difference vector:
# Difference vector 1: closed - open diff.vec.1 <- difference.vector(pdbs$xyz[c(1,5), gaps.pos$f.inds]) # Difference vector 2: closed - rstate diff.vec.2 <- difference.vector(pdbs$xyz[c(5,9), gaps.pos$f.inds]) # Calculate overlap oa <- overlap(modes.open, diff.vec.1) ob <- overlap(modes.closed, diff.vec.1) oc <- overlap(modes.closed, diff.vec.2)
plot(oa$overlap.cum[1:10], type='b', ylim=c(0,1), ylab="Squared overlap", xlab="Mode index", lwd=2) lines(ob$overlap.cum[1:10], type='b', lty=2, col=2, lwd=2) lines(oc$overlap.cum[1:10], type='b', lty=3, col=4, lwd=1) legend("bottomright", c("Open to closed", "Closed to open", "Closed to r-state"), col=c(1,2,4), lty=c(1,2,3))
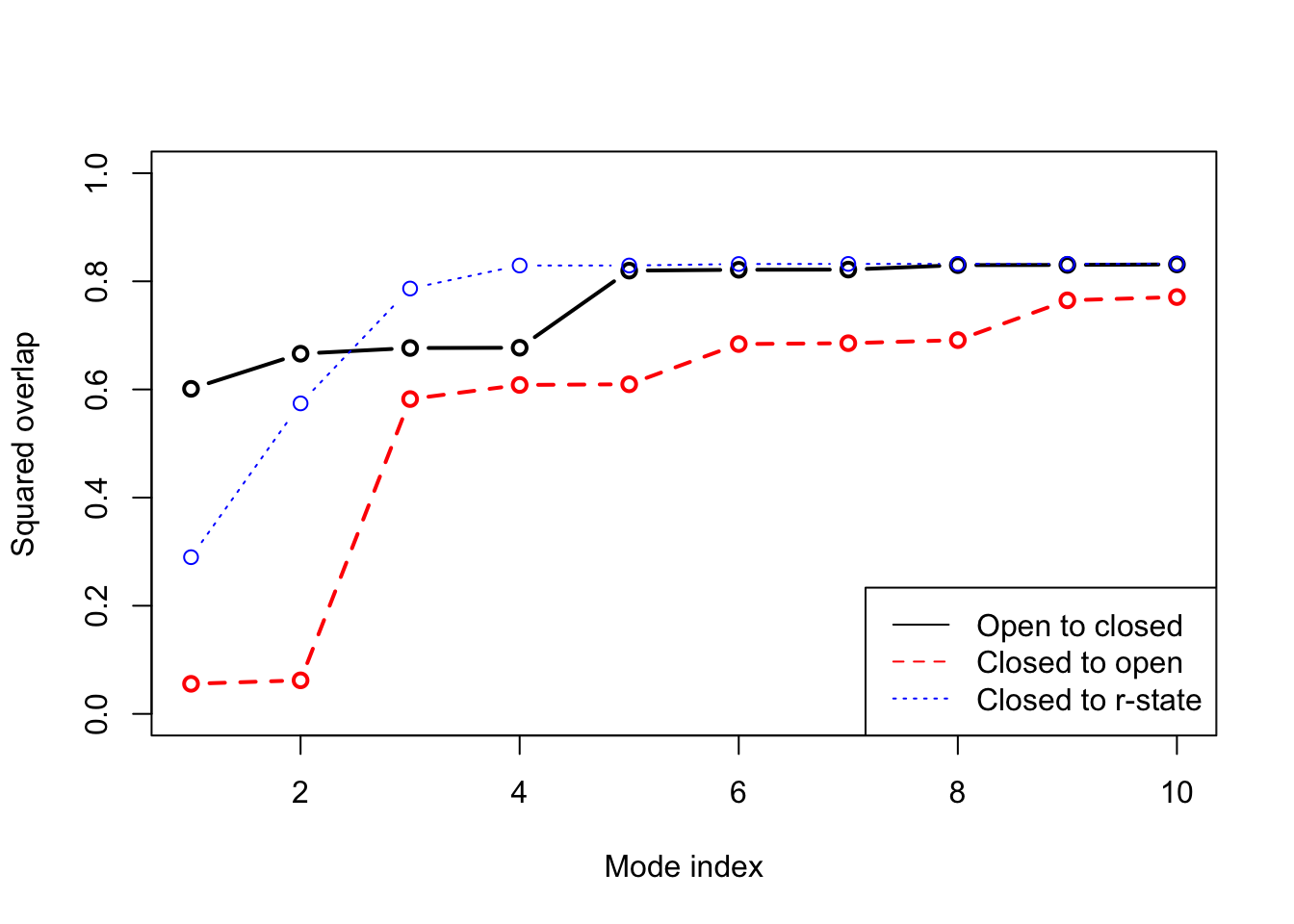
Overlap anlaysis with function overlap(). The modes calculated on the open state of the GroEL subunit shows a high similarity to the conformational difference vector (black), while the agreement is lower when the normal modes are calculated on the closed state (red). Blue line correspond to the overlap between the closed state and the r-state (a semi-open state characterized by a rotation of the apical domain in the opposite direction as compared to the open state.
Variance weighting
From the overlap analysis above we see the good agreement (high overlap value) between the conformational difference vector and the normal modes calculated on the open structures. Contrary, the lowest frequency modes of the closed structures does not show the same behavior. We will thus proceed with the weighting of the force constants. First we will calculate the variance of all pairwise distances in the ensemble and use this as weights for the NMA:
# Calcualte variance wts <- var.xyz(pdbs$xyz[, gaps.pos$f.inds])
Weights to the force constants can be included by the argument ‘fc.weights’ to function nma(). This needs be a matrix with dimensions NxN (where N is the number of C-alpha atoms). Here we will run a small for-loop with increasing the strength of the weighting at each step and store the new overlap values in the variable ‘ob.wtd’:
ob.wtd <- NULL for ( i in 1:10 ) { modes.wtd <- nma(pdb.closed, fc.weights=wts**i) ob.tmp <- overlap(modes.wtd, diff.vec.1) ob.wtd <- rbind(ob.wtd, ob.tmp$overlap.cum) }
plot(oa$overlap.cum[1:10], type='b', ylim=c(0,1), ylab="Squared overlap", xlab="Mode index", axes=T, lwd=2) lines(ob$overlap.cum[1:10], type='b', lty=2, col=1, lwd=2) cols <- rainbow(10) for ( i in 1:nrow(ob.wtd) ) { lines(ob.wtd[i,1:10], type='b', lty=1, col=cols[i]) } legend("bottomright", c("Open state", "Closed state", "Closed state (weighted)"), col=c("black", "black", "green"), lty=c(1,2,1))
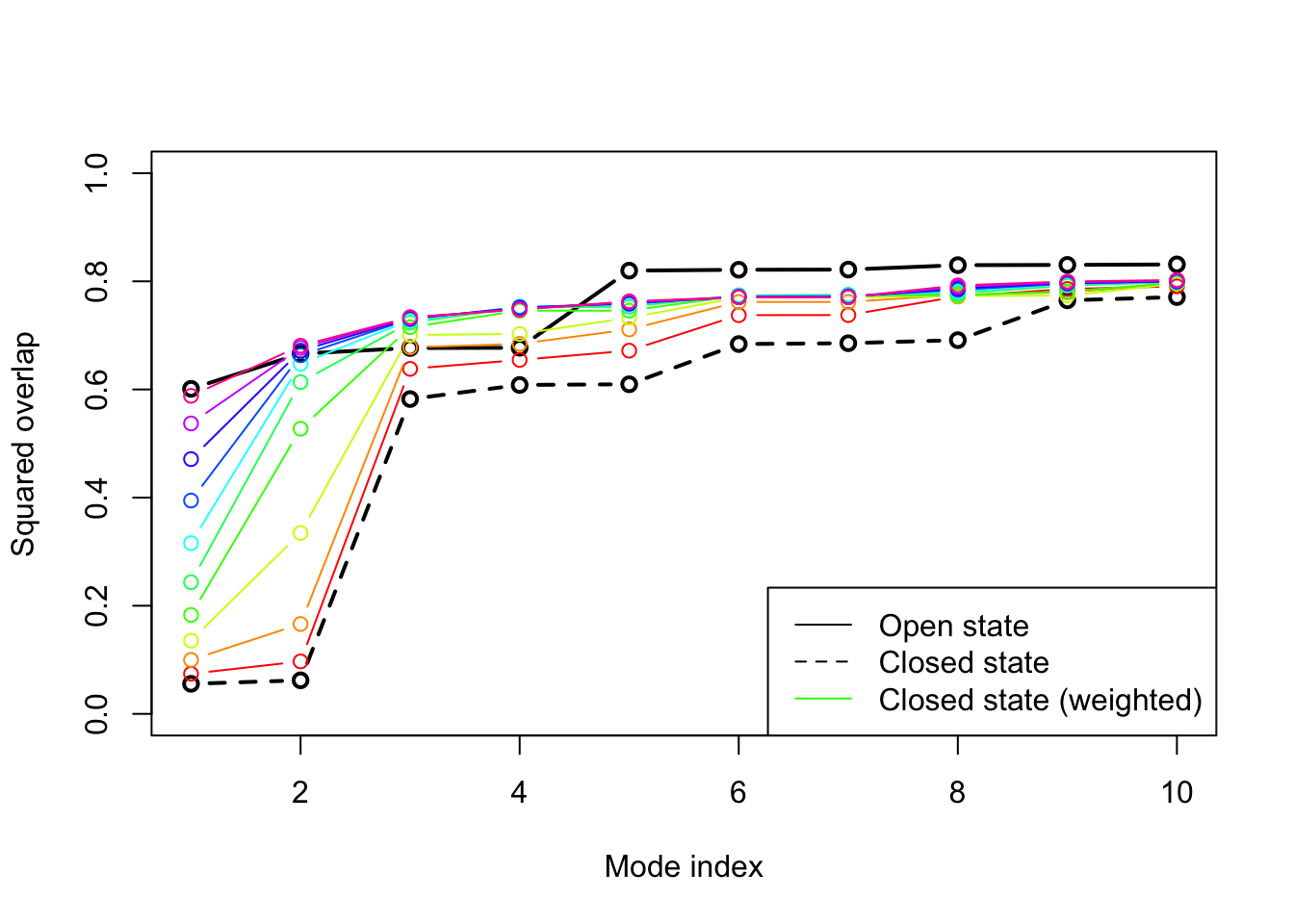
Overlap plot with increasing strength on the weighting. The final weighted normal modes of the closed subunit shows as high overlap values as the modes for the open state.
RMSIP calculation
RMSIP can be used to compare the mode subspaces:
par(mfrow=c(1,2)) plot(ra, ylab="NMA(open)", xlab="NMA(weighted)") plot(rb, ylab="NMA(open)", xlab="NMA(closed)")
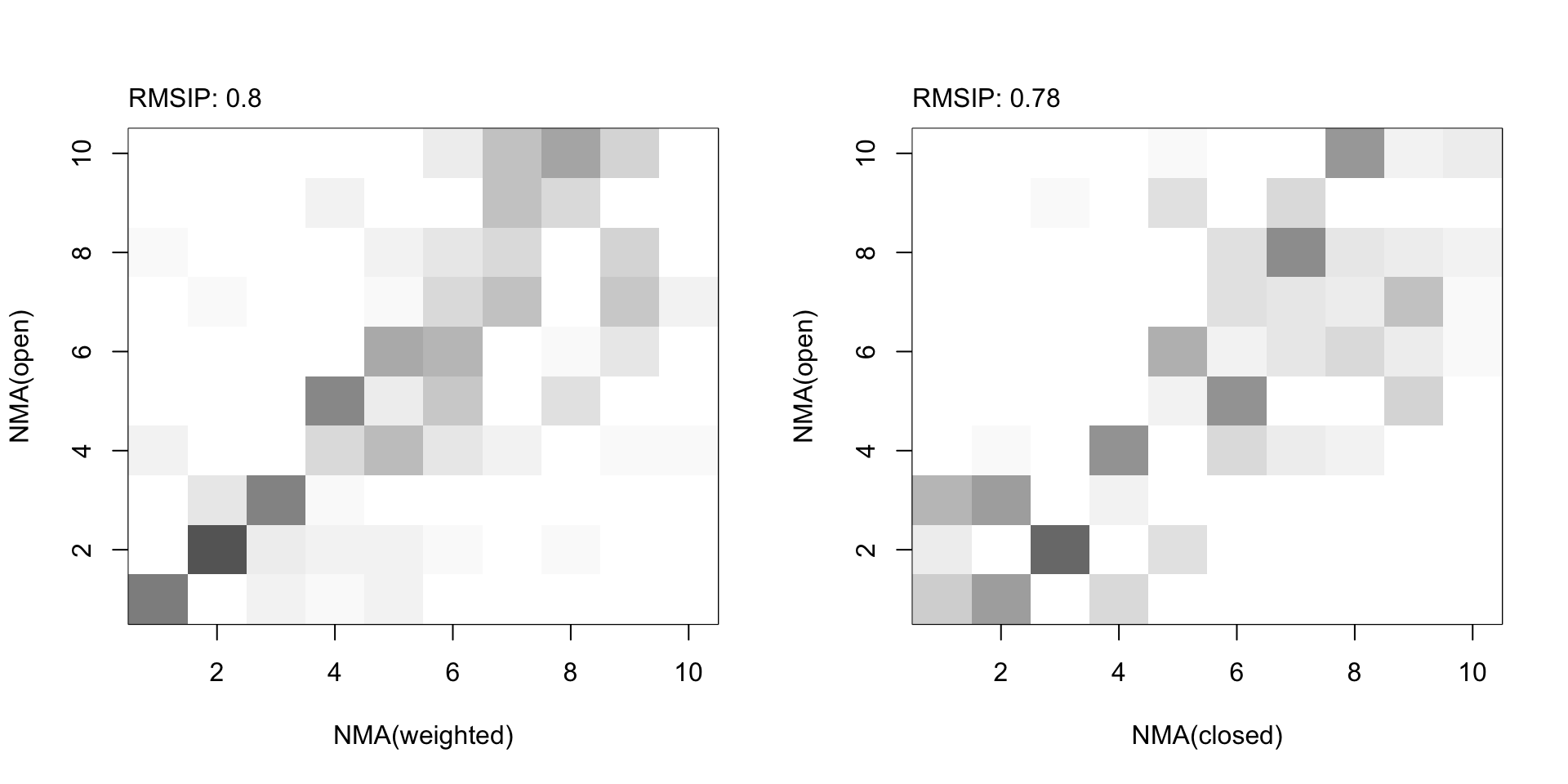
RMSIP maps between (un)weighted normal modes obtained from the open and closed subunits.
Match with PCA
Finally, we compare the calculated normal modes with principal components obtained from the ensemble of X-ray structures using function pca.xyz():
# Calculate the PCs pc.xray <- pca.xyz(pdbs$xyz[,gaps.pos$f.inds])
# or alternatively... pc.xray <- pca(pdbs) # Calculate RMSIP values rmsip(pc.xray, modes.open)$rmsip
## [1] 0.6208435rmsip(pc.xray, modes.closed)$rmsip
## [1] 0.630455rmsip(pc.xray, modes.rstate)$rmsip
## [1] 0.586484rmsip(pc.xray, modes.wtd)$rmsip
## [1] 0.6606216Example 4B: Transducin
This example will run nma() on transducin with variance weighted force constants. The modes predicted by NMA will be compared with principal component analysis (PCA) results over the transducin family. We load the transducin data via the command data(transducin) and calculate the normal modes for two structures corresponding to two nucleotide states, respectively: GDP (PDB id 1TAG) and GTP (PDB id 1TND). Again we use function pdbs2pdb() to build the pdb objects from the pdbs object (containing aligned structure/sequence information). The coordinates of the data set were fitted to all non-gap containing C-alpha positions.
data(transducin) pdbs <- transducin$pdbs gaps.res <- gap.inspect(pdbs$ali) gaps.pos <- gap.inspect(pdbs$xyz)
# Fit coordinates based on all non-gap positions # and do PCA xyz <- pdbfit(pdbs) pc.xray <- pca.xyz(xyz[, gaps.pos$f.inds])
# Fetch PDB objects npdbs <- pdbs npdbs$xyz <- xyz pdb.list <- pdbs2pdb(npdbs, inds=c(2, 7), rm.gaps=TRUE) pdb.gdp <- pdb.list[[ grep("1TAG_A", names(pdb.list)) ]] pdb.gtp <- pdb.list[[ grep("1TND_B", names(pdb.list)) ]] # Calculate normal modes modes.gdp <- nma(pdb.gdp) modes.gtp <- nma(pdb.gtp)
Now, we calculate the pairwise distance variance based on the structural ensemble with the function var.xyz() as described above. This will be used to weight the force constants in the elastic network model.
# Calculate weights weights <- var.xyz(xyz[, gaps.pos$f.inds]) # Calculate normal modes with weighted pair force constants modes.gdp.b <- nma(pdb.gdp, fc.weights=weights**100) modes.gtp.b <- nma(pdb.gtp, fc.weights=weights**100)
To evaluate the results, we calculate the overlap (square dot product) between modes predicted by variance weighted or non-weighted NMA and the first principal component from PCA.
oa <- overlap(modes.gdp, pc.xray$U[,1]) ob <- overlap(modes.gtp, pc.xray$U[,1]) oc <- overlap(modes.gdp.b, pc.xray$U[,1]) od <- overlap(modes.gtp.b, pc.xray$U[,1])
plot(oa$overlap.cum, type='o', ylim=c(0,1), col="darkgreen", lwd=2, xlab="Mode", ylab="Cummulative overlap") lines(ob$overlap.cum, type='o', ylim=c(0,1), col="red", lwd=2) lines(oc$overlap.cum, type='b', ylim=c(0,1), col="darkgreen", lwd=2, lty=2) lines(od$overlap.cum, type='b', ylim=c(0,1), col="red", lwd=2, lty=2) text(20, oa$overlap.cum[20], label=round(oa$overlap.cum[20], 2), pos=3) text(20, ob$overlap.cum[20], label=round(ob$overlap.cum[20], 2), pos=3) text(20, oc$overlap.cum[20], label=round(oc$overlap.cum[20], 2), pos=3) text(20, od$overlap.cum[20], label=round(od$overlap.cum[20], 2), pos=3) legend("topleft", pch=1, lty=c(1, 1, 2, 2), col=c("darkgreen", "red", "darkgreen", "red"), legend=c("GDP", "GTP", "Weighted GDP", "Weighted GTP"))
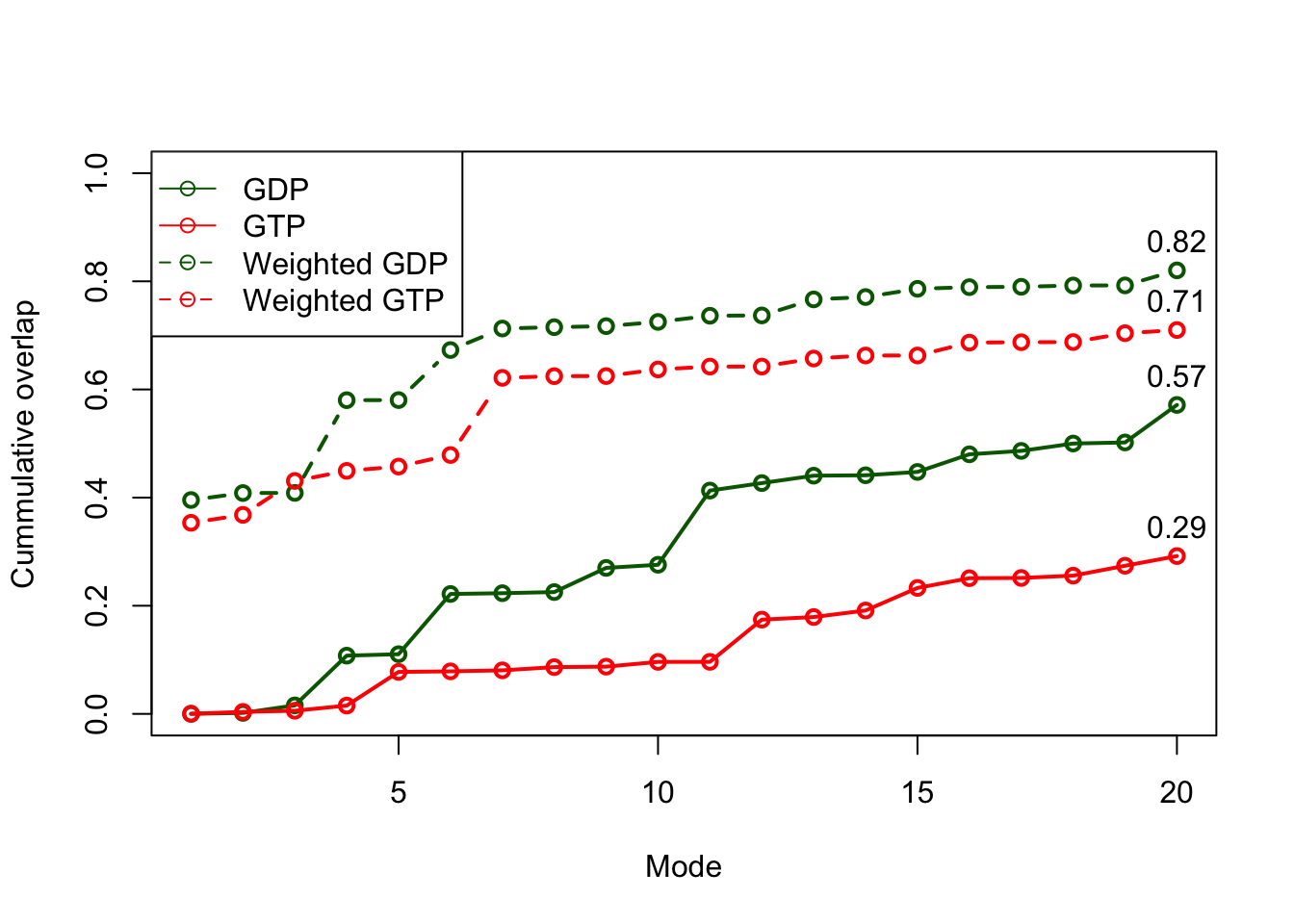
Variance weighted force constants improve NMA prediction
Example 5: User-defined pair force constant functions
In this example we demonstrate the interface for defining custom functions for the pair spring force constants. A custom function can be obtained through simple scripting as shown below.
Example 5A: Specifying a simple function
We first show how to define a simple force constant function by building a revised version of the parameter-free ANM force field. The function my.ff() below takes as input r which is a vector of inter-atomic (calpha) distances (i.e. distances from atom i, to all other atoms in the system; this function will thus be called N times, where N is the number of calpha atoms). It will in this case return 0 for the pairs with a distance larger than 10 , and \(r^{-2}\) for all other pairs. Our simple function will thus look like:
# Define function for spring force constants "my.ff" <- function(r, ...) { ifelse( r>10, 0, r^(-2) ) }
Once the function is in place we can feed it to function nma() to calculate the normal modes based on the particular force constants built with our new function. Below we apply it to the lysozyme structure (PDB id 1hel) from Example 1:
Alternatively we can take a more manual approach by calling build.hessian() if we want to investigate the Hessian matrix further (note that build.hessian is called from within function nma() which will diagonalize the hessian to obtain the normal modes and thus not return it to the user). In the code below we first build the hessian and illustrate how to obtain the normal modes through calls to either eigen() or nma() (which can also take a Hessian matrix as input):
# Indices for CA atoms ca.inds <- atom.select(pdb, 'calpha') # Build hessian matrix h <- build.hessian(pdb$xyz[ ca.inds$xyz ], pfc.fun=my.ff) # Diagonalize and obtain eigenvectors and eigenvalues modes <- eigen(h, symmetric=TRUE) # ... or feed the Hessian to function 'nma()' modes <- nma(pdb, hessian=h, mass=FALSE)
Note that function nma() assumes the Hessian to be mass-weighted and we therefore have to specify mass=FALSE in this particular case. To obtain a mass-weighted Hessian pass the amino acid masses through argument aa.mass to function build.hessian().
Example 5B: Specific force constants for disulfide bridges
In the following code we illustrate a more advanced force constant function making use of arguments atom.id and ssdat which is passed from function build.hessian() by default. This allows users to access the protein sequence (ssdat$seq), secondary structure data (ssdat$sse), beta bridges (ssdat$beta.bridges), helix 1-4 (ssdat$helix14), and disulfide bridges (ss bonds; ssdat$ss.bonds) when building the force constants.
First we define our new function (ff.custom()) and specify the force constants which should be applied to bonded and non-bonded interactions (k.bonded and k.nonbonded, respectively). Next we define the the force constant for the disulfide bridges (k.ssbond):
"ff.custom" <- function(r, atom.id, pdb=NULL, ss.bonds=NULL, ...) { # Default force constants (Hinsen et al 2000) k.bonded <- (r * 8.6 * 10^2) - (2.39 * 10^3) k.nonbonded <- (128 * 10^4) * r^(-6) # Special force constant for SS-bonds k.ssbond <- 50; # Calculate default values (equivalent to the calpha ff) ks <- ifelse(r < 4.0, k.bonded, k.nonbonded) if(!is.null(ss.bonds)) { # If atom.id is part off a ssbond.. inds <- which(ss.bonds == atom.id, arr.ind=TRUE) if(length(inds) > 0) { # Find ss-bond pair inds.paired <- ss.bonds[ inds[1, "row"], ] j <- inds.paired[ inds.paired != atom.id ] # and change the spring force constant ks[j] <- k.ssbond } } return(ks) }
The disulfide bridges can be supplied as input to nma() function via a simple two-column matrix:
Document Details
This document is shipped with the Bio3D package in both R and PDF formats. All code can be extracted and automatically executed to generate Figures and/or the PDF with the following commands:
Information About the Current Bio3D Session
## R version 3.6.0 (2019-04-26)
## Platform: x86_64-apple-darwin15.6.0 (64-bit)
## Running under: macOS 10.15.5
##
## Matrix products: default
## BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
## LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
##
## locale:
## [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] bio3d_2.4-1.9000
##
## loaded via a namespace (and not attached):
## [1] Rcpp_1.0.5 lattice_0.20-41 digest_0.6.25 crayon_1.3.4
## [5] rprojroot_1.3-2 assertthat_0.2.1 grid_3.6.0 R6_2.4.1
## [9] backports_1.1.8 magrittr_1.5 evaluate_0.14 highr_0.8
## [13] stringi_1.4.6 rlang_0.4.6.9000 fs_1.4.2 rmarkdown_2.3.2
## [17] pkgdown_1.5.1.9000 desc_1.2.0 tools_3.6.0 stringr_1.4.0
## [21] parallel_3.6.0 yaml_2.2.1 xfun_0.15 compiler_3.6.0
## [25] memoise_1.1.0 htmltools_0.5.0 knitr_1.29References
Durand, P., G. Trinquier, and Y. H. Sanejouand. 1994. “A New Approach for Determining Low-Frequency Normal Modes in Macromolecules.” Biopolymers 34: 759–71. https://doi.org/10.1002/bip.360340608.
Fuglebakk, Edvin, Julián Echave, and Nathalie Reuter. 2012. “Measuring and comparing structural fluctuation patterns in large protein datasets.” Bioinformatics (Oxford, England) 28 (19): 2431–40. https://doi.org/10.1093/bioinformatics/bts445.
Grant, B. J., A. P. D. C Rodrigues, K. M. Elsawy, A. J. Mccammon, and L. S. D. Caves. 2006. “Bio3d: An R Package for the Comparative Analysis of Protein Structures.” Bioinformatics 22: 2695–6. https://doi.org/10.1093/bioinformatics/btl461.
Hinsen, K, A J Petrescu, S Dellerue, M C Bellissent-Funel, and G R Kneller. 2000. “Harmonicity in slow protein dynamics.” Chemical Physics 261 (1-2): 25–37. https://doi.org/10.1016/S0301-0104(00)00222-6.
Romanowska, Julia, Krzysztof S. Nowinski, and Joanna Trylska. 2012. “Determining geometrically stable domains in molecular conformation sets.” Journal of Chemical Theory and Computation 8 (8): 2588–99. https://doi.org/10.1021/ct300206j.
Skjaerven, L., X. Q. Yao, G. Scarabelli, and B. J. Grant. 2015. “Integrating Protein Structural Dynamics and Evolutionary Analysis with Bio3d.” BMC Bioinformatics 15: 399. https://doi.org/10.1186/s12859-014-0399-6.
Tama, F., F. X. Gadea, O. Marques, and Y. H. Sanejouand. 2000. “Building-Block Approach for Determining Low-Frequency Normal Modes of Macromolecules.” Proteins 41: 1–7. https://doi.org/10.1002/1097-0134(20001001)41:1<1::AID-PROT10>3.0.CO;2-P.
Tama, F, and Y H Sanejouand. 2001. “Conformational change of proteins arising from normal mode calculations.” Protein Eng 14 (1): 1–6. https://doi.org/10.1093/protein/14.1.1.
Yao, X. Q., L. Skjaerven, and B. J. Grant. 2016. “Rapid Characterization of Allosteric Networks with Ensemble Normal Mode Analysis.” The Journal of Physical Chemistry B 120: 8276–88. https://doi.org/10.1021/acs.jpcb.6b01991.
The latest version of the package, full documentation and further vignettes (including detailed installation instructions) can be obtained from the main Bio3D website: http://thegrantlab.org/bio3d/.↩︎
This vignette contains executable examples, see
help(vignette)for further details.↩︎See also dedicated vignettes for ensemble NMA provided with the Bio3D package.↩︎